[ad_1]
New electrode material preserves its volume, solving a major limitation of solid-state batteries
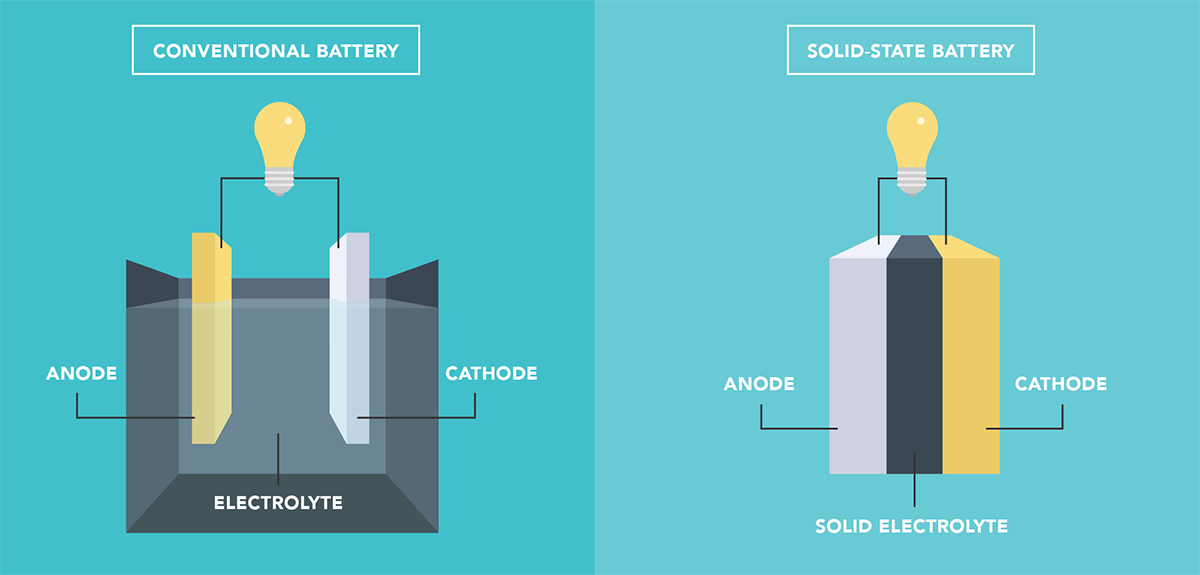
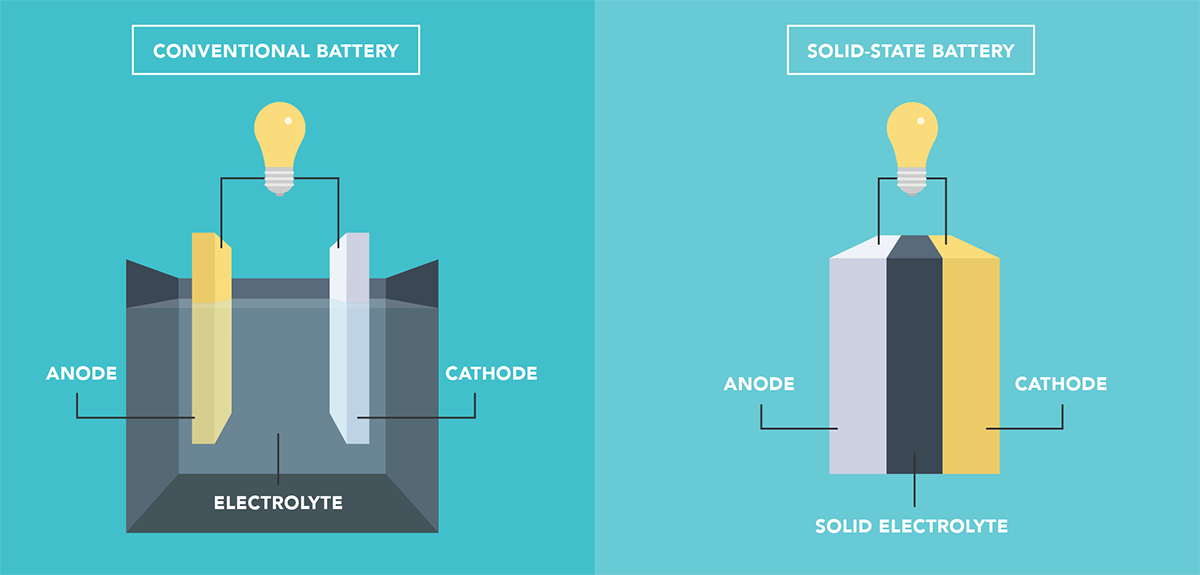
Solid-state batteries (SSBs) offer several potential advantages over current lithium-ion batteries, including improved safety and faster charging. However, SSBs suffer from a problem that limits their durability. When lithium ions are inserted into or extracted from the electrodes, the crystalline structure of the material changes, making the electrode expand or shrink. These repeated changes in volume damage the interface between the electrodes and the solid electrolyte and cause irreversible alterations in the crystal chemistry of the electrodes.
Now, a team of scientists led by Professor Naoaki Yabuuchi of Yokohama National University has investigated a new type of cathode material that could provide unprecedented stability in SSBs. The team describes its work in “A near dimensionally invariable high-capacity positive electrode material,” published in nature materials.
The material the research team focused on was Li8/7Ti2/7V4/7O2, a binary system composed of optimized portions of lithium titanate (Li2TiO3) and lithium vanadium dioxide (LiVO2). When ball-milled down to an appropriate particle size in the order of nanometers, this material offers high capacity thanks to its large quantity of lithium ions that can be reversibly inserted and extracted during the charge/discharge process.
Unlike other positive electrode materials, Li8/7Ti2/7V4/7O2 has nearly the same volume when fully charged and discharged. During delithiation, some vanadium ions migrate from their original position to the spaces left behind by the lithium ions, acquiring a higher oxidation state in the process. This causes a repulsive interaction with oxygen, which in turn produces an expansion of the crystal lattice.
“When shrinkage and expansion are well balanced, dimensional stability is retained while the battery is charged or discharged,” Professor Yabuuchi says. “We anticipate that a truly dimensionally invariable material—one that retains its volume upon electrochemical cycling—could be developed by further optimizing the chemical composition of the electrolyte.”
The research team tested this new positive electrode material in a solid-state cell, and the cell exhibited a capacity of 300 mAh/g with no degradation over 400 charge/discharge cycles.
“The absence of capacity fading over 400 cycles clearly indicates the superior performance of this material compared with those reported for conventional all-solid-state cells with layered materials,” said study co-author Professor Neeraj Sharma. “This finding could drastically reduce battery costs.”
Source: EurekAlert!
[ad_2]
Source link